S. Korea starts administering dementia-delaying drug “Lecanemab”
입력 2024.12.22 (01:24)
읽어주기 기능은 크롬기반의
브라우저에서만 사용하실 수 있습니다.
[Anchor]
Following the United States, Japan, and China, hospitals in our country have now also begun prescribing a new drug that slows the progression of dementia.
What are the effects and limitations of this dementia drug?
Medical reporter Park Kwang-sik tells us.
[Report]
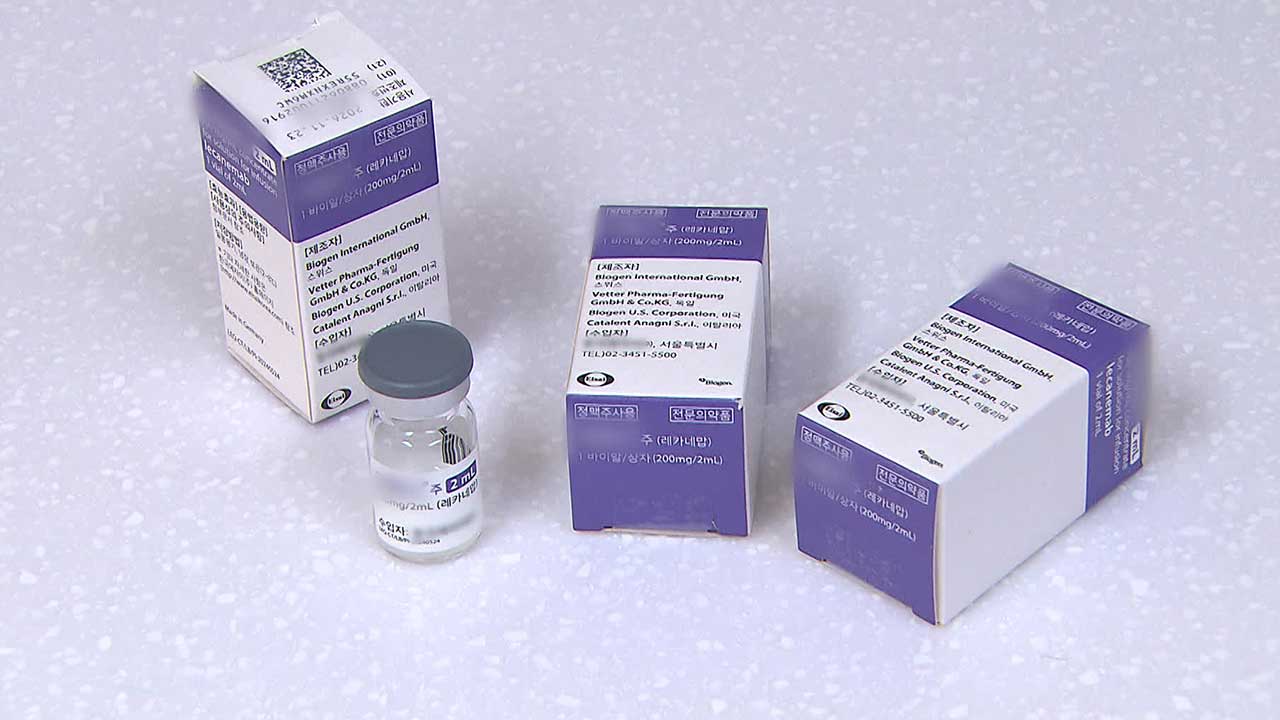
This dementia drug containing the ingredient 'Lecanemab' was jointly developed by the United States and Japan, and has been introduced domestically starting this month.
It is an antibody treatment that fundamentally removes 'dementia-causing substances' in the brain.
A 70-year-old dementia patient, who is about to receive the first dose, has high expectations.
[Lee ○○/Mild Cognitive Impairment Patient: "Rather than significantly improvement (of cognitive function), can it not stop the progression? Even that would be so good. It would be very good."]
While existing dementia medications only temporarily alleviate symptoms, this new drug has been shown to delay the progression of dementia by an average of 27%.
Assuming 10 years remaining of a person's life, this means delaying the progression of dementia by about 30 months.
However, it is disappointing that even if 'dementia-causing substances' are removed, it does not lead to a complete cure.
This is because the causes of dementia are diverse, and it is difficult to recover already damaged nerve cells.
Accordingly, the treatment target for this new drug is limited to patients with mild cognitive impairment or early-stage dementia, who have had 'dementia-causing proteins' detected in their brain.
Additionally, side effects such as brain hemorrhage or brain edema may occur during the treatment process, which makes regular brain MRI scans necessary.
[Kang Seong-hoon/Professor of Neurology at Korea University Guro Hospital: "When amyloid (dementia-causing protein) is broken down, these fragments can affect the blood vessels in the brain, weakening the walls of the blood vessels. If the walls of the blood vessels are weakened, even a slight rupture can lead to a brain hemorrhage..."]
Moreover, the treatment involves biweekly injections over a period of one and a half years, costing about 40 million won.
The high treatment cost and the fact that it is not a cure are issues that need to be further addressed.
Experts emphasize that in a situation where 1 in 10 elderly people suffer from dementia, delaying the progression by just 1 to 2 years can significantly reduce social costs, such as caregiving burdens.
This is KBS News, Park Kwang-sik.
Following the United States, Japan, and China, hospitals in our country have now also begun prescribing a new drug that slows the progression of dementia.
What are the effects and limitations of this dementia drug?
Medical reporter Park Kwang-sik tells us.
[Report]
This dementia drug containing the ingredient 'Lecanemab' was jointly developed by the United States and Japan, and has been introduced domestically starting this month.
It is an antibody treatment that fundamentally removes 'dementia-causing substances' in the brain.
A 70-year-old dementia patient, who is about to receive the first dose, has high expectations.
[Lee ○○/Mild Cognitive Impairment Patient: "Rather than significantly improvement (of cognitive function), can it not stop the progression? Even that would be so good. It would be very good."]
While existing dementia medications only temporarily alleviate symptoms, this new drug has been shown to delay the progression of dementia by an average of 27%.
Assuming 10 years remaining of a person's life, this means delaying the progression of dementia by about 30 months.
However, it is disappointing that even if 'dementia-causing substances' are removed, it does not lead to a complete cure.
This is because the causes of dementia are diverse, and it is difficult to recover already damaged nerve cells.
Accordingly, the treatment target for this new drug is limited to patients with mild cognitive impairment or early-stage dementia, who have had 'dementia-causing proteins' detected in their brain.
Additionally, side effects such as brain hemorrhage or brain edema may occur during the treatment process, which makes regular brain MRI scans necessary.
[Kang Seong-hoon/Professor of Neurology at Korea University Guro Hospital: "When amyloid (dementia-causing protein) is broken down, these fragments can affect the blood vessels in the brain, weakening the walls of the blood vessels. If the walls of the blood vessels are weakened, even a slight rupture can lead to a brain hemorrhage..."]
Moreover, the treatment involves biweekly injections over a period of one and a half years, costing about 40 million won.
The high treatment cost and the fact that it is not a cure are issues that need to be further addressed.
Experts emphasize that in a situation where 1 in 10 elderly people suffer from dementia, delaying the progression by just 1 to 2 years can significantly reduce social costs, such as caregiving burdens.
This is KBS News, Park Kwang-sik.
■ 제보하기
▷ 카카오톡 : 'KBS제보' 검색, 채널 추가
▷ 전화 : 02-781-1234, 4444
▷ 이메일 : kbs1234@kbs.co.kr
▷ 유튜브, 네이버, 카카오에서도 KBS뉴스를 구독해주세요!
- S. Korea starts administering dementia-delaying drug “Lecanemab”
-
- 입력 2024-12-22 01:24:47
[Anchor]
Following the United States, Japan, and China, hospitals in our country have now also begun prescribing a new drug that slows the progression of dementia.
What are the effects and limitations of this dementia drug?
Medical reporter Park Kwang-sik tells us.
[Report]
This dementia drug containing the ingredient 'Lecanemab' was jointly developed by the United States and Japan, and has been introduced domestically starting this month.
It is an antibody treatment that fundamentally removes 'dementia-causing substances' in the brain.
A 70-year-old dementia patient, who is about to receive the first dose, has high expectations.
[Lee ○○/Mild Cognitive Impairment Patient: "Rather than significantly improvement (of cognitive function), can it not stop the progression? Even that would be so good. It would be very good."]
While existing dementia medications only temporarily alleviate symptoms, this new drug has been shown to delay the progression of dementia by an average of 27%.
Assuming 10 years remaining of a person's life, this means delaying the progression of dementia by about 30 months.
However, it is disappointing that even if 'dementia-causing substances' are removed, it does not lead to a complete cure.
This is because the causes of dementia are diverse, and it is difficult to recover already damaged nerve cells.
Accordingly, the treatment target for this new drug is limited to patients with mild cognitive impairment or early-stage dementia, who have had 'dementia-causing proteins' detected in their brain.
Additionally, side effects such as brain hemorrhage or brain edema may occur during the treatment process, which makes regular brain MRI scans necessary.
[Kang Seong-hoon/Professor of Neurology at Korea University Guro Hospital: "When amyloid (dementia-causing protein) is broken down, these fragments can affect the blood vessels in the brain, weakening the walls of the blood vessels. If the walls of the blood vessels are weakened, even a slight rupture can lead to a brain hemorrhage..."]
Moreover, the treatment involves biweekly injections over a period of one and a half years, costing about 40 million won.
The high treatment cost and the fact that it is not a cure are issues that need to be further addressed.
Experts emphasize that in a situation where 1 in 10 elderly people suffer from dementia, delaying the progression by just 1 to 2 years can significantly reduce social costs, such as caregiving burdens.
This is KBS News, Park Kwang-sik.
Following the United States, Japan, and China, hospitals in our country have now also begun prescribing a new drug that slows the progression of dementia.
What are the effects and limitations of this dementia drug?
Medical reporter Park Kwang-sik tells us.
[Report]
This dementia drug containing the ingredient 'Lecanemab' was jointly developed by the United States and Japan, and has been introduced domestically starting this month.
It is an antibody treatment that fundamentally removes 'dementia-causing substances' in the brain.
A 70-year-old dementia patient, who is about to receive the first dose, has high expectations.
[Lee ○○/Mild Cognitive Impairment Patient: "Rather than significantly improvement (of cognitive function), can it not stop the progression? Even that would be so good. It would be very good."]
While existing dementia medications only temporarily alleviate symptoms, this new drug has been shown to delay the progression of dementia by an average of 27%.
Assuming 10 years remaining of a person's life, this means delaying the progression of dementia by about 30 months.
However, it is disappointing that even if 'dementia-causing substances' are removed, it does not lead to a complete cure.
This is because the causes of dementia are diverse, and it is difficult to recover already damaged nerve cells.
Accordingly, the treatment target for this new drug is limited to patients with mild cognitive impairment or early-stage dementia, who have had 'dementia-causing proteins' detected in their brain.
Additionally, side effects such as brain hemorrhage or brain edema may occur during the treatment process, which makes regular brain MRI scans necessary.
[Kang Seong-hoon/Professor of Neurology at Korea University Guro Hospital: "When amyloid (dementia-causing protein) is broken down, these fragments can affect the blood vessels in the brain, weakening the walls of the blood vessels. If the walls of the blood vessels are weakened, even a slight rupture can lead to a brain hemorrhage..."]
Moreover, the treatment involves biweekly injections over a period of one and a half years, costing about 40 million won.
The high treatment cost and the fact that it is not a cure are issues that need to be further addressed.
Experts emphasize that in a situation where 1 in 10 elderly people suffer from dementia, delaying the progression by just 1 to 2 years can significantly reduce social costs, such as caregiving burdens.
This is KBS News, Park Kwang-sik.
-
-

박광식 기자 doctor@kbs.co.kr
박광식 기자의 기사 모음
-
이 기사가 좋으셨다면
-
좋아요
0
-
응원해요
0
-
후속 원해요
0










![[속보] 정성호 “검찰 해체 표현 부적절…국민 요구사항, 검사들도 잘 알 것”](/data/layer/904/2025/07/20250701_86evyp.png)



이 기사에 대한 의견을 남겨주세요.