CLINICAL TRIAL FOR ANTIBODY TREATMENT
입력 2021.01.14 (15:04)
수정 2021.01.14 (16:45)
읽어주기 기능은 크롬기반의
브라우저에서만 사용하실 수 있습니다.
[Anchor Lead]
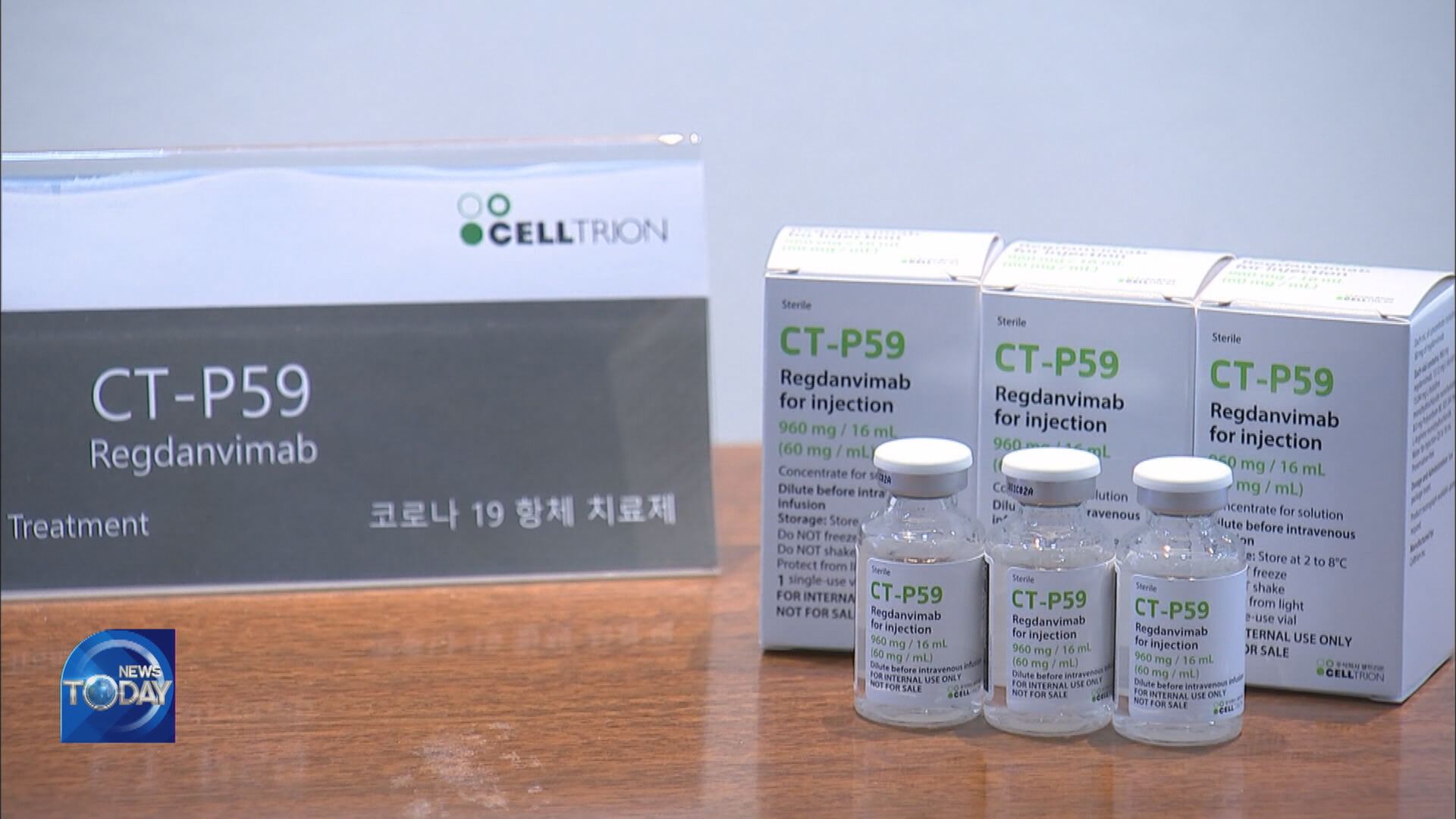
Data of Phase 2 clinical trials for Korea's first antibody treatment for COVID-19 has been disclosed. The treatment has been found to prevent the disease from deteriorating in 54 percent of patients with mild and moderate symptoms, and significantly shorten their recovery period. Although it's effective in treating coronavirus at the early stages, the treatment is believed to have limited effects in treating seriously ill patients who have sustained organ damage due to COVID-19.
[Pkg]
Korean pharmaceutical firm Celltrion is developing an antibody treatment for COVID-19. The results of Phase 2 clinical trials conducted on some 300 patients with mild and moderate symptoms revealed that patients who received the treatment were 54 percent less likely to see their condition deteriorate compared to the placebo group. Pneumonia patients aged 50 and up were found to be 68 percent less likely to become gravely ill. This means the antibody can effectively prevent conditions from worsening in COVID-19 patients with mild and moderate symptoms when administered at an early stage. It also shortens the recovery time from various symptoms such as fever, cough and difficulty in breathing by up to 6.4 days.
[Soundbite] EOM JOONG SIK(PROF., GACHON UNIVERSITY) : "This treatment could help alleviate the burden of medical centers when finding ICU beds and increase hospital capacity in treating high-risk patients."
However, some experts say the antibody treatment has limited effects in critically ill patients. The treatment blocks the virus from further attacking the body by attaching to the spike protein, where the virus binds with human cells. However, when symptoms reach the severe phase, excessive inflammation is a more serious problem than the virus itself.
[Soundbite] KIM WOO-JOO(PROF., KOREA UNIVERSITY GURO HOSPITAL) : "The antibody treatment works in patients with moderate symptoms, but it's not effective in ICU patients."
There were no severe reactions or deaths during the clinical trials. If Celltrion's antibody treatment is sufficiently verified in terms of safety and efficacy, the Ministry of Food and Drug Safety plans to issue a permit, but under one condition: the company must later submit the results of Phase 3 clinical trials.
Data of Phase 2 clinical trials for Korea's first antibody treatment for COVID-19 has been disclosed. The treatment has been found to prevent the disease from deteriorating in 54 percent of patients with mild and moderate symptoms, and significantly shorten their recovery period. Although it's effective in treating coronavirus at the early stages, the treatment is believed to have limited effects in treating seriously ill patients who have sustained organ damage due to COVID-19.
[Pkg]
Korean pharmaceutical firm Celltrion is developing an antibody treatment for COVID-19. The results of Phase 2 clinical trials conducted on some 300 patients with mild and moderate symptoms revealed that patients who received the treatment were 54 percent less likely to see their condition deteriorate compared to the placebo group. Pneumonia patients aged 50 and up were found to be 68 percent less likely to become gravely ill. This means the antibody can effectively prevent conditions from worsening in COVID-19 patients with mild and moderate symptoms when administered at an early stage. It also shortens the recovery time from various symptoms such as fever, cough and difficulty in breathing by up to 6.4 days.
[Soundbite] EOM JOONG SIK(PROF., GACHON UNIVERSITY) : "This treatment could help alleviate the burden of medical centers when finding ICU beds and increase hospital capacity in treating high-risk patients."
However, some experts say the antibody treatment has limited effects in critically ill patients. The treatment blocks the virus from further attacking the body by attaching to the spike protein, where the virus binds with human cells. However, when symptoms reach the severe phase, excessive inflammation is a more serious problem than the virus itself.
[Soundbite] KIM WOO-JOO(PROF., KOREA UNIVERSITY GURO HOSPITAL) : "The antibody treatment works in patients with moderate symptoms, but it's not effective in ICU patients."
There were no severe reactions or deaths during the clinical trials. If Celltrion's antibody treatment is sufficiently verified in terms of safety and efficacy, the Ministry of Food and Drug Safety plans to issue a permit, but under one condition: the company must later submit the results of Phase 3 clinical trials.
■ 제보하기
▷ 카카오톡 : 'KBS제보' 검색, 채널 추가
▷ 전화 : 02-781-1234, 4444
▷ 이메일 : kbs1234@kbs.co.kr
▷ 유튜브, 네이버, 카카오에서도 KBS뉴스를 구독해주세요!
- CLINICAL TRIAL FOR ANTIBODY TREATMENT
-
- 입력 2021-01-14 15:04:04
- 수정2021-01-14 16:45:25
[Anchor Lead]
Data of Phase 2 clinical trials for Korea's first antibody treatment for COVID-19 has been disclosed. The treatment has been found to prevent the disease from deteriorating in 54 percent of patients with mild and moderate symptoms, and significantly shorten their recovery period. Although it's effective in treating coronavirus at the early stages, the treatment is believed to have limited effects in treating seriously ill patients who have sustained organ damage due to COVID-19.
[Pkg]
Korean pharmaceutical firm Celltrion is developing an antibody treatment for COVID-19. The results of Phase 2 clinical trials conducted on some 300 patients with mild and moderate symptoms revealed that patients who received the treatment were 54 percent less likely to see their condition deteriorate compared to the placebo group. Pneumonia patients aged 50 and up were found to be 68 percent less likely to become gravely ill. This means the antibody can effectively prevent conditions from worsening in COVID-19 patients with mild and moderate symptoms when administered at an early stage. It also shortens the recovery time from various symptoms such as fever, cough and difficulty in breathing by up to 6.4 days.
[Soundbite] EOM JOONG SIK(PROF., GACHON UNIVERSITY) : "This treatment could help alleviate the burden of medical centers when finding ICU beds and increase hospital capacity in treating high-risk patients."
However, some experts say the antibody treatment has limited effects in critically ill patients. The treatment blocks the virus from further attacking the body by attaching to the spike protein, where the virus binds with human cells. However, when symptoms reach the severe phase, excessive inflammation is a more serious problem than the virus itself.
[Soundbite] KIM WOO-JOO(PROF., KOREA UNIVERSITY GURO HOSPITAL) : "The antibody treatment works in patients with moderate symptoms, but it's not effective in ICU patients."
There were no severe reactions or deaths during the clinical trials. If Celltrion's antibody treatment is sufficiently verified in terms of safety and efficacy, the Ministry of Food and Drug Safety plans to issue a permit, but under one condition: the company must later submit the results of Phase 3 clinical trials.
Data of Phase 2 clinical trials for Korea's first antibody treatment for COVID-19 has been disclosed. The treatment has been found to prevent the disease from deteriorating in 54 percent of patients with mild and moderate symptoms, and significantly shorten their recovery period. Although it's effective in treating coronavirus at the early stages, the treatment is believed to have limited effects in treating seriously ill patients who have sustained organ damage due to COVID-19.
[Pkg]
Korean pharmaceutical firm Celltrion is developing an antibody treatment for COVID-19. The results of Phase 2 clinical trials conducted on some 300 patients with mild and moderate symptoms revealed that patients who received the treatment were 54 percent less likely to see their condition deteriorate compared to the placebo group. Pneumonia patients aged 50 and up were found to be 68 percent less likely to become gravely ill. This means the antibody can effectively prevent conditions from worsening in COVID-19 patients with mild and moderate symptoms when administered at an early stage. It also shortens the recovery time from various symptoms such as fever, cough and difficulty in breathing by up to 6.4 days.
[Soundbite] EOM JOONG SIK(PROF., GACHON UNIVERSITY) : "This treatment could help alleviate the burden of medical centers when finding ICU beds and increase hospital capacity in treating high-risk patients."
However, some experts say the antibody treatment has limited effects in critically ill patients. The treatment blocks the virus from further attacking the body by attaching to the spike protein, where the virus binds with human cells. However, when symptoms reach the severe phase, excessive inflammation is a more serious problem than the virus itself.
[Soundbite] KIM WOO-JOO(PROF., KOREA UNIVERSITY GURO HOSPITAL) : "The antibody treatment works in patients with moderate symptoms, but it's not effective in ICU patients."
There were no severe reactions or deaths during the clinical trials. If Celltrion's antibody treatment is sufficiently verified in terms of safety and efficacy, the Ministry of Food and Drug Safety plans to issue a permit, but under one condition: the company must later submit the results of Phase 3 clinical trials.
이 기사가 좋으셨다면
-
좋아요
0
-
응원해요
0
-
후속 원해요
0












![[단독] 위성락 실장 “전작권 협상 카드 아냐”…차관 인선 발표](/data/layer/904/2025/07/20250713_krfuHu.jpg)



이 기사에 대한 의견을 남겨주세요.