SPECIAL MEETING ON VACCINATION PLANS
입력 2021.04.13 (15:25)
수정 2021.04.13 (16:45)
읽어주기 기능은 크롬기반의
브라우저에서만 사용하실 수 있습니다.
[Anchor Lead]
Amid fears of another surge in COVID-19 infections, President Moon Jae-in on Monday presided over a special meeting on quarantine inspection, pointing to the gravity of the situation. The government has also unveiled vaccine production plans to take place at home.
[Pkg]
The Seoul government has for the first time outlined a timetable on introducing the Novavax COVID-19 vaccine. At the time the contract was signed in February, it was only announced that the entire doses for 20 million people would be produced in Korea to be distributed in phases from the second quarter. According to the latest details, production is set to begin this month. Once authorities grant approval in the year's first half, the completed vaccine is expected to roll out from as early as June. Thereafter, Novavax vaccines for ten million people will be supplied through the third quarter.
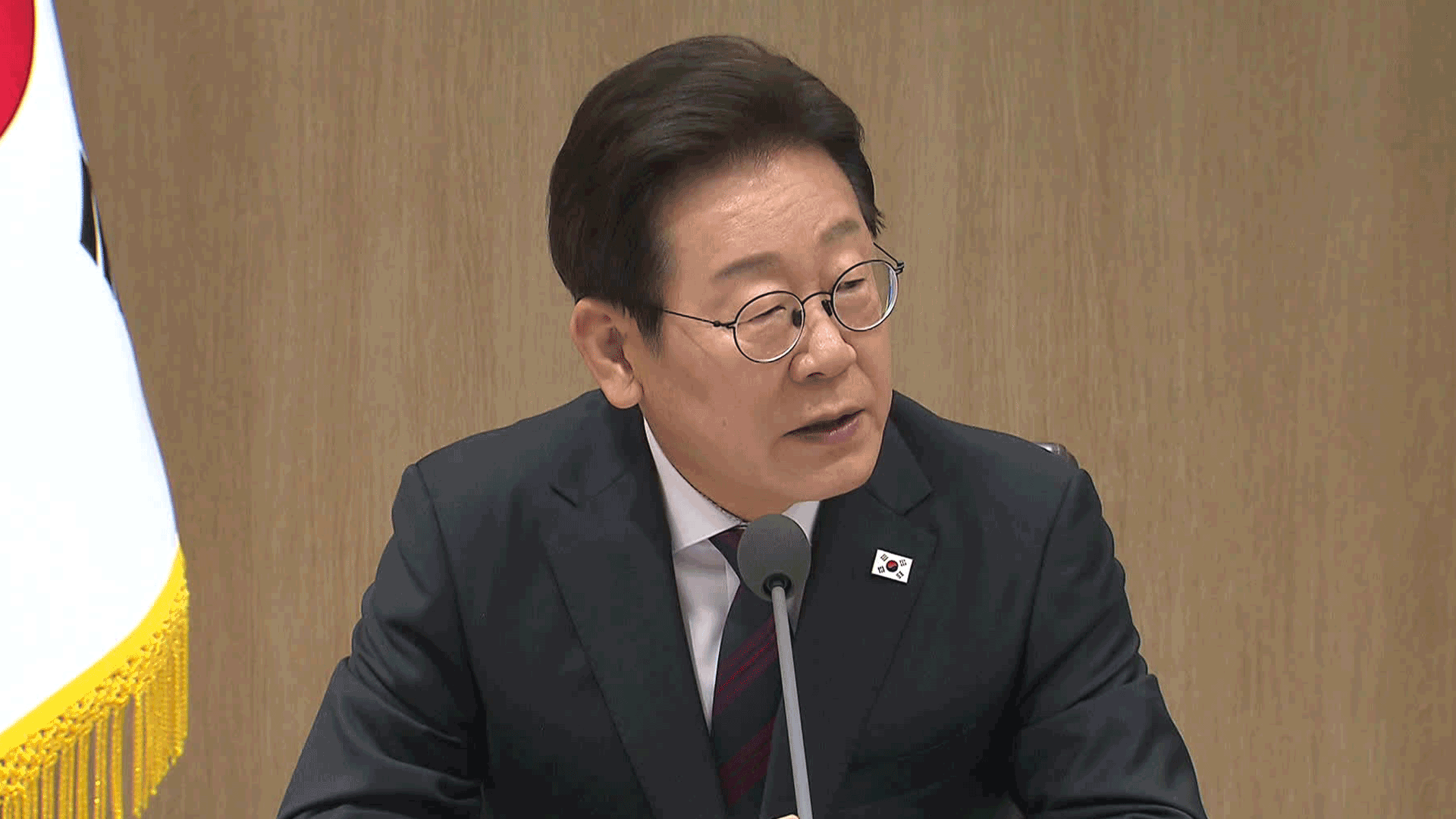
[Soundbite] (Pres. Moon Jae-in) : "The foundation has been laid out for a stable supply of domestically produced vaccine. I ask officials to exert full administrative and diplomatic efforts for the swift introduction and further procurement of vaccines."
The government explained there was initially a supply delay due to setbacks in securing the raw materials caused by U.S. export regulations. But the issue has been mostly settled now after Seoul sought direct cooperation with drug firms.
[Soundbite] Kwon Deok-cheol(Minister of Health & Welfare) : "Amid growing uncertainties around global vaccine supplies, production at home will help Korea stabilize the inoculation process."
Inoculations are also likely to begin from as early as June.
[Soundbite] Jung Eun-kyeong(KDCA Director) : "Domestic production schedule will be pushed up and permit issues will be quickly sorted out to enable vaccinations at the earliest date."
South Korea will introduce the Novavax vaccine supplies enough for 20 million people in total, marking the first case of a consignment production taking place at home through technology transfer. COVID-19 vaccines for 7.7 million people are so far set to arrive during the second quarter from individual pharmaceutical firms and the COVAX global procurement program. This volume consists of the Pfizer vaccine for over 3 million people and the AstraZeneca vaccine for 4.5 million. If Novavax is added to this total, the vaccination program can further pick up speed.
[Soundbite] Lee Jae-Gap(Hallym Univ. Kangnam Sacred Heart Hospital) : "The Novavax vaccine can be stored at regular refrigerator temperature and therefore it's an important vaccine to ramp up the speed of vaccinations."
Meanwhile the Food and Drug Safety Ministry has launched a review process to authorize another vaccine developed by Moderna after the US firm filed for a permit.
Amid fears of another surge in COVID-19 infections, President Moon Jae-in on Monday presided over a special meeting on quarantine inspection, pointing to the gravity of the situation. The government has also unveiled vaccine production plans to take place at home.
[Pkg]
The Seoul government has for the first time outlined a timetable on introducing the Novavax COVID-19 vaccine. At the time the contract was signed in February, it was only announced that the entire doses for 20 million people would be produced in Korea to be distributed in phases from the second quarter. According to the latest details, production is set to begin this month. Once authorities grant approval in the year's first half, the completed vaccine is expected to roll out from as early as June. Thereafter, Novavax vaccines for ten million people will be supplied through the third quarter.
[Soundbite] (Pres. Moon Jae-in) : "The foundation has been laid out for a stable supply of domestically produced vaccine. I ask officials to exert full administrative and diplomatic efforts for the swift introduction and further procurement of vaccines."
The government explained there was initially a supply delay due to setbacks in securing the raw materials caused by U.S. export regulations. But the issue has been mostly settled now after Seoul sought direct cooperation with drug firms.
[Soundbite] Kwon Deok-cheol(Minister of Health & Welfare) : "Amid growing uncertainties around global vaccine supplies, production at home will help Korea stabilize the inoculation process."
Inoculations are also likely to begin from as early as June.
[Soundbite] Jung Eun-kyeong(KDCA Director) : "Domestic production schedule will be pushed up and permit issues will be quickly sorted out to enable vaccinations at the earliest date."
South Korea will introduce the Novavax vaccine supplies enough for 20 million people in total, marking the first case of a consignment production taking place at home through technology transfer. COVID-19 vaccines for 7.7 million people are so far set to arrive during the second quarter from individual pharmaceutical firms and the COVAX global procurement program. This volume consists of the Pfizer vaccine for over 3 million people and the AstraZeneca vaccine for 4.5 million. If Novavax is added to this total, the vaccination program can further pick up speed.
[Soundbite] Lee Jae-Gap(Hallym Univ. Kangnam Sacred Heart Hospital) : "The Novavax vaccine can be stored at regular refrigerator temperature and therefore it's an important vaccine to ramp up the speed of vaccinations."
Meanwhile the Food and Drug Safety Ministry has launched a review process to authorize another vaccine developed by Moderna after the US firm filed for a permit.
■ 제보하기
▷ 카카오톡 : 'KBS제보' 검색, 채널 추가
▷ 전화 : 02-781-1234, 4444
▷ 이메일 : kbs1234@kbs.co.kr
▷ 유튜브, 네이버, 카카오에서도 KBS뉴스를 구독해주세요!
- SPECIAL MEETING ON VACCINATION PLANS
-
- 입력 2021-04-13 15:25:02
- 수정2021-04-13 16:45:37
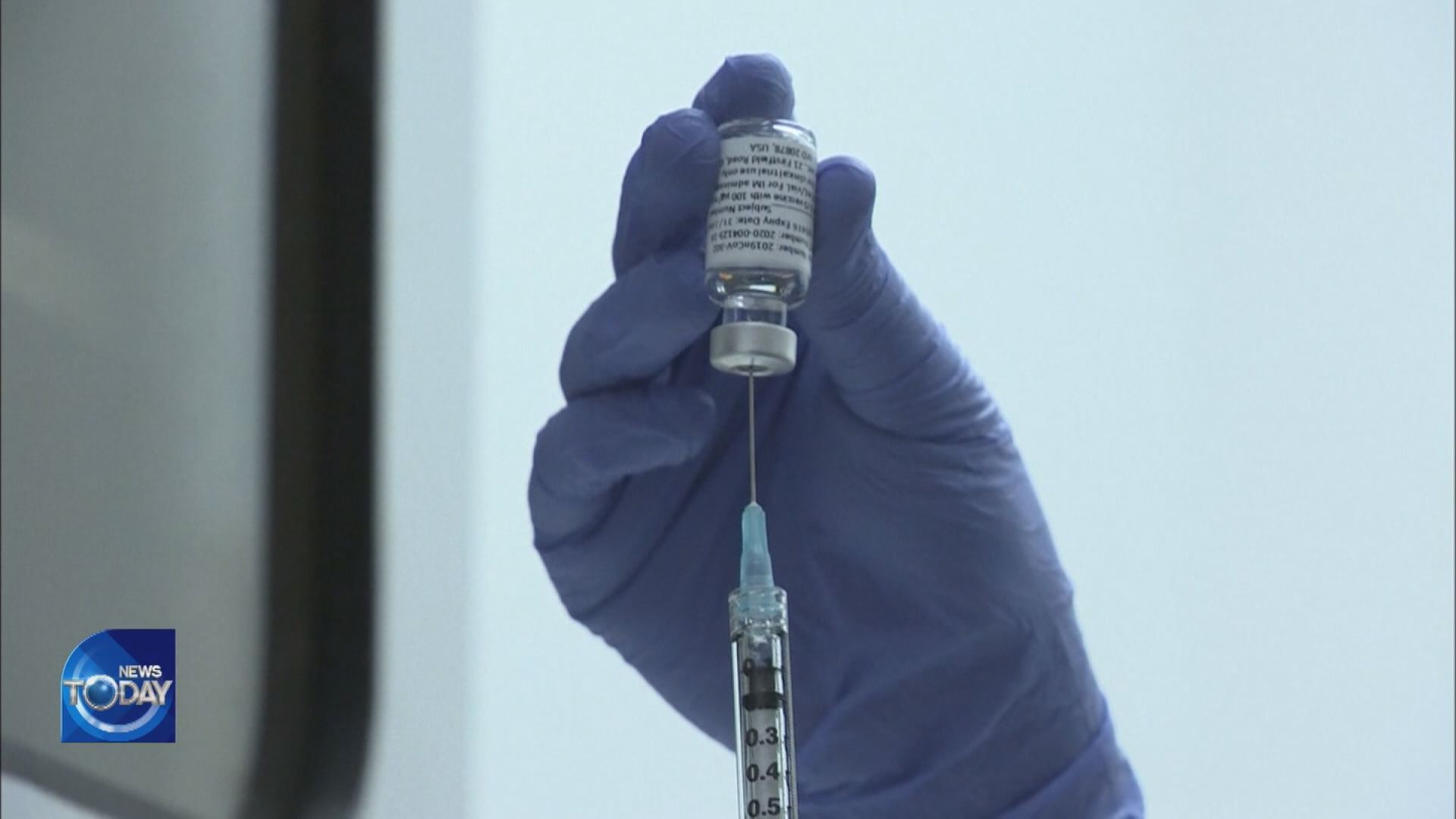
[Anchor Lead]
Amid fears of another surge in COVID-19 infections, President Moon Jae-in on Monday presided over a special meeting on quarantine inspection, pointing to the gravity of the situation. The government has also unveiled vaccine production plans to take place at home.
[Pkg]
The Seoul government has for the first time outlined a timetable on introducing the Novavax COVID-19 vaccine. At the time the contract was signed in February, it was only announced that the entire doses for 20 million people would be produced in Korea to be distributed in phases from the second quarter. According to the latest details, production is set to begin this month. Once authorities grant approval in the year's first half, the completed vaccine is expected to roll out from as early as June. Thereafter, Novavax vaccines for ten million people will be supplied through the third quarter.
[Soundbite] (Pres. Moon Jae-in) : "The foundation has been laid out for a stable supply of domestically produced vaccine. I ask officials to exert full administrative and diplomatic efforts for the swift introduction and further procurement of vaccines."
The government explained there was initially a supply delay due to setbacks in securing the raw materials caused by U.S. export regulations. But the issue has been mostly settled now after Seoul sought direct cooperation with drug firms.
[Soundbite] Kwon Deok-cheol(Minister of Health & Welfare) : "Amid growing uncertainties around global vaccine supplies, production at home will help Korea stabilize the inoculation process."
Inoculations are also likely to begin from as early as June.
[Soundbite] Jung Eun-kyeong(KDCA Director) : "Domestic production schedule will be pushed up and permit issues will be quickly sorted out to enable vaccinations at the earliest date."
South Korea will introduce the Novavax vaccine supplies enough for 20 million people in total, marking the first case of a consignment production taking place at home through technology transfer. COVID-19 vaccines for 7.7 million people are so far set to arrive during the second quarter from individual pharmaceutical firms and the COVAX global procurement program. This volume consists of the Pfizer vaccine for over 3 million people and the AstraZeneca vaccine for 4.5 million. If Novavax is added to this total, the vaccination program can further pick up speed.
[Soundbite] Lee Jae-Gap(Hallym Univ. Kangnam Sacred Heart Hospital) : "The Novavax vaccine can be stored at regular refrigerator temperature and therefore it's an important vaccine to ramp up the speed of vaccinations."
Meanwhile the Food and Drug Safety Ministry has launched a review process to authorize another vaccine developed by Moderna after the US firm filed for a permit.
Amid fears of another surge in COVID-19 infections, President Moon Jae-in on Monday presided over a special meeting on quarantine inspection, pointing to the gravity of the situation. The government has also unveiled vaccine production plans to take place at home.
[Pkg]
The Seoul government has for the first time outlined a timetable on introducing the Novavax COVID-19 vaccine. At the time the contract was signed in February, it was only announced that the entire doses for 20 million people would be produced in Korea to be distributed in phases from the second quarter. According to the latest details, production is set to begin this month. Once authorities grant approval in the year's first half, the completed vaccine is expected to roll out from as early as June. Thereafter, Novavax vaccines for ten million people will be supplied through the third quarter.
[Soundbite] (Pres. Moon Jae-in) : "The foundation has been laid out for a stable supply of domestically produced vaccine. I ask officials to exert full administrative and diplomatic efforts for the swift introduction and further procurement of vaccines."
The government explained there was initially a supply delay due to setbacks in securing the raw materials caused by U.S. export regulations. But the issue has been mostly settled now after Seoul sought direct cooperation with drug firms.
[Soundbite] Kwon Deok-cheol(Minister of Health & Welfare) : "Amid growing uncertainties around global vaccine supplies, production at home will help Korea stabilize the inoculation process."
Inoculations are also likely to begin from as early as June.
[Soundbite] Jung Eun-kyeong(KDCA Director) : "Domestic production schedule will be pushed up and permit issues will be quickly sorted out to enable vaccinations at the earliest date."
South Korea will introduce the Novavax vaccine supplies enough for 20 million people in total, marking the first case of a consignment production taking place at home through technology transfer. COVID-19 vaccines for 7.7 million people are so far set to arrive during the second quarter from individual pharmaceutical firms and the COVAX global procurement program. This volume consists of the Pfizer vaccine for over 3 million people and the AstraZeneca vaccine for 4.5 million. If Novavax is added to this total, the vaccination program can further pick up speed.
[Soundbite] Lee Jae-Gap(Hallym Univ. Kangnam Sacred Heart Hospital) : "The Novavax vaccine can be stored at regular refrigerator temperature and therefore it's an important vaccine to ramp up the speed of vaccinations."
Meanwhile the Food and Drug Safety Ministry has launched a review process to authorize another vaccine developed by Moderna after the US firm filed for a permit.
이 기사가 좋으셨다면
-
좋아요
0
-
응원해요
0
-
후속 원해요
0










![[HEADLINE]](https://news.kbs.co.kr/data/news/title_image/newsmp4/news_today/2021/04/13/10_5161390.jpeg)




이 기사에 대한 의견을 남겨주세요.